Emergency Contraception: Increasing Public Awareness
Emergency contraception has the potential to greatly reduce the number of unintended pregnancies in the United States. However, that potential is largely unrealized because most women are unaware that a back-up method is available and most health care providers do not routinely discuss emergency contraception with their patients. To address this situation, medical and public health groups have recently launched targeted awareness initiatives, and policymakers in Congress have introduced legislation to fund a nationwide public education campaign.
About half of the 6.3 million pregnancies every year in the United States are unintended, and more than half of those end in abortion. Despite their differing positions on abortion, prochoice and antiabortion advocates agree that these proportions are too high. It is remarkable then that emergency contraception—one of the most important tools for reducing unintended pregnancy—remains so unknown and inaccessible. Many women have not heard of emergency contraception, and even those who report that they have often confuse it with the abortion pill, mifepristone. Yet, best estimates indicate that if emergency contraceptives were widely available in the United States, 1.7 million unintended pregnancies could be avoided, and the number of abortions each year could be cut by as much as half.
This article begins by addressing misperceptions that emergency contraception is something new and untested or inherently unsafe, and that it is comparable to an abortion. It then describes efforts that are underway to increase awareness among consumers and health care providers alike.
What Is Emergency Contraception?
Emergency contraception, or postcoital contraception, as it is more properly known, consists of the same hormones found in ordinary birth control pills.* When taken in a concentrated dose shortly after unprotected intercourse, these hormones can prevent pregnancy from occurring. As such, postcoital contraception is considered a "back-up" birth control option for occasional use.
Hormonal postcoital contraception dates back to the mid-1960s, when a Dutch family planning physician provided high-dose estrogens to a 13-year-old rape victim. This treatment became standard during the 1960s and early 1970s. Beginning in the 1970s, the high-dose estrogen therapy gave way to a combined estrogen-progestin regimen known as the Yuzpe method, named for the Canadian physician A. Albert Yuzpe, who developed it using ordinary birth control pills. At around the same time, researchers began investigating the safety and efficacy of a regimen consisting of a progestin (levonorgestrel) alone, and found that it could be as effective as the Yuzpe regimen.
Both the Yuzpe method and the levonorgestrel-only emergency contraceptive regimen consist of two doses of pills to be initiated within 72 hours after unprotected intercourse. Given the low levels of hormone and short duration of exposure, these methods are considered to be safe for nearly every woman. In addition, there are no known risk factors associated with emergency contraception, either for the woman or for the fetus, if the pills are taken accidentally during pregnancy. About half of the women who use the Yuzpe regimen experience nausea, and about one in five experience vomiting; some also report breast tenderness, fatigue and headaches similar to what might be encountered with ongoing oral contraceptive use. The incidence of side effects among women using levonorgestrel alone is significantly lower: About one in four women report nausea, and about one in 18 report vomiting.
Research suggests that emergency contraception reduces the pregnancy risk of a woman who has had unprotected intercourse by about 75%. Emergency contraception prevents pregnancy in the same way as other hormonal contraceptive methods, such as the pill, the injectable (Depo-Provera) and even breastfeeding: by delaying or inhibiting ovulation, inhibiting fertilization or inhibiting implantation of a fertilized egg, depending on when during the menstrual cycle a woman initiates the method. (According to the National Institutes of Health, the Food and Drug Administration (FDA) and the American College of Obstetricians and Gynecologists (ACOG), pregnancy begins when a fertilized egg implants in the lining of the uterus.) Thus, emergency contraception is no more a "do-it-yourself abortion kit" than are regular birth control pills; it has no effect once a pregnancy has been established.
Bringing to Market
As early as the 1970s, emergency contraception became widely available in some European countries, and in 1984, the United Kingdom became the first country to approve a product specifically packaged as emergency contraception. Today, dedicated products are registered in over 80 countries worldwide (see box). In the United States, the FDA did not approve a dedicated product until 1998. Before that time, doctors who prescribed emergency contraception had to do so "off label," by cutting up packages of oral contraceptives with the appropriate hormones. While this is a legal and medically accepted practice, many physicians failed to provide information about emergency contraception to women during routine visits, since they did not have a labeled and marketed product. Not surprisingly, many women were unaware that the method was available, according to a 1997 Kaiser Family Foundation survey.
| Worldwide Availability Countries with Dedicated Products Approved for Emergency Contraception | |
|---|---|
|
Africa and the Middle East Algeria Benin Cameroon Dem. Rep. of the Congo Egypt Gabon Guinea Israel Ivory Coast Kenya Madagascar Mali Mauritania Mauritius Morocco Namibia Republic of the Congo Senegal South Africa Tunisia Uganda Yemen Zimbabwe Asia and Oceania Armenia Azerbaijan Bangladesh China Fiji Georgia India Kazakhstan Kyrgyzstan New Zealand South Korea Sri Lanka Taiwan Tajikistan Thailand Turkmenistan Uzbekistan Vietnam |
Americas Argentina Brazil Canada Columbia Dominican Republic El Salvador Jamaica Mexico Paraguay Peru United States Uruguay Venezuela Europe Austria Belarus Belgium Bulgaria Czech Republic Denmark Estonia Finland France Germany Greece Iceland Italy Luxembourg Moldova Netherlands Norway Portugal Romania Russia Slovakia Spain Sweden Ukraine United Kingdom |
| Source: The International Consortium for Emergency Contraception, http://www.cecinfo.org, as of August 30, 2002. | |
In an attempt to bring emergency contraception into the medical mainstream, the Center for Reproductive Law and Policy filed a citizen petition with the FDA in 1994 on behalf of a coalition of public health and medical groups. The petition requested the FDA to require large pharmaceutical companies to provide information for postcoital emergency contraception on oral contraceptive packaging and labels. The FDA declined to exercise its authority in this way, but took the unusual step in 1997 of issuing a notice in the Federal Register declaring emergency contraception to be safe and effective, and encouraging manufacturers to apply for approval of a dedicated product. In 1998 and 1999, two such products were approved: Preven, consisting of the Yuzpe method, and Plan B, consisting of the levonorgestrel-only emergency contraception regimen.
The Nation's 'Best Kept Secret'
In part because dedicated products have been available for a longer period of time in Europe, knowledge of emergency contraception is greater there than in the United States: Studies estimate 75-95% awareness in Europe among adolescents, women in general and women seeking termination of a pregnancy. For example, a nationally representative study of more than 4,000 teens in Switzerland, published in the July 2002 issue of the Journal of Adolescent Health, shows that most sexually active young women (89%) and young men (75%) know about emergency contraception.
The European experience also demonstrates the importance of public and provider education campaigns for improving awareness. In the United Kingdom, awareness of emergency contraception was low in 1984, the year the method was approved there: Just 12% of women seeking termination of pregnancy had good knowledge of postcoital contraceptives. Since the late 1980s, there have been several campaigns—largely government-funded—aimed at improving awareness as a way of addressing the problem of unintended pregnancy, especially among teens. The largest of these was launched in 1995 by the Health Education Authority, which invested nearly $2.5 million over three years for a campaign consisting of a 24-hour hot line and advertisements on the radio and in women's magazines and health professional journals. Data show that by 1996, awareness had climbed to 76% among women seeking termination of pregnancy.
Similar to the U.K. experience, awareness of emergency contraception in the United States was low before the advent of a dedicated product; however, U.S. awareness continues to lag behind that of Europe. According to a November 2000 survey conducted by the Kaiser Family Foundation and Lifetime Television, 51% of U.S. women aged 18-44 say that there is something a woman can do in the few days after she has had unprotected sex to prevent pregnancy. However, many of those who had heard that some sort of method exists did not know that a product is available in the United States, that it requires a prescription or when after sex it needs to be taken to be effective.
Moreover, various studies indicate that many U.S. women confuse emergency contraception with the abortion pill, mifepristone. Antiabortion and anti-family planning activists, who believe that life begins after fertilization, have deliberately confused the two drugs by equating the use of emergency contraceptives with abortion. "Long before implantation, there is a real human being living inside the mother's body," writes Elizabeth Bossom of Concerned Women for America. "People are being deceived when they are told the [emergency contraceptive] pill is not an abortifacient." While some antiabortion organizations (such as the American Life League) are forthright in their opposition not just to emergency contraception but to oral contraception as well, most shy away from the argument that emergency contraception is no different in its effect than ordinary birth control.
Getting the Word Out
Key actors in the medical and public health communities and in the companies that make emergency contraception have recently taken steps to get the word out to two important constituencies: providers and women. According to a nationally representative survey of health care providers conducted by the Kaiser Family Foundation in 2000, only one in five obstetrician-gynecologists discuss emergency contraception as part of their routine counseling (see chart a). While this is a significant improvement from the proportion in 1997, the data indicate that providers still have not integrated emergency contraception into their routine care.
| chart a Discussion Lacking |
|---|
| The proportions of obstetrician-gynecologists and family practice physicians who routinely discuss emergency contraception have increased in recent years but remain small. |
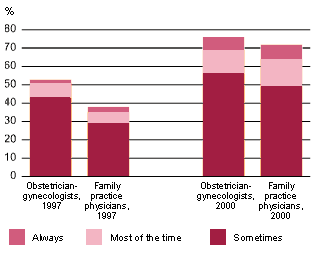 |
|
Source: Third National Survey of Women's Health Care Providers on Reproductive Health, Questions and detailed results: emergency contraception, Kaiser Family Foundation, 2000. |
In an attempt to involve more obstetrician-gynecologists in the drive to reduce the number of unintended pregnancies, ACOG issued a call to action in April 2001 to the nation's 40,000 obstetrician-gynecologists, asking them to proactively discuss emergency contraception with women and to offer advance prescriptions for the method. The call to action was followed in February 2002 by a mailing to ACOG members that again urged them to talk to their patients and to encourage pharmacists to stock Plan B and Preven. "If most women had emergency contraception in their medicine cabinet, or a prescription for it, we could help cut the U.S. rate of unintended pregnancy in half," said past-president of ACOG Thomas Purdon in the call to action.
Despite ACOG's efforts, convincing doctors in private practice to discuss emergency contraception remains a challenge. According to James Trussell, Director of the Office of Population Research at Princeton University, "Most do not deliberately hold back information for moral reasons. Physicians believe that the method is safe and effective." Drawing a sharp distinction between family planning clinics and private doctors' offices, Sharon Camp, CEO of the company that makes Plan B, speculates that many physicians in private practice do not routinely discuss the back-up method "because they are not as seized with the need to reduce unintended pregnancy." She believes that there may also be attitudinal barriers that keep physicians from discussing emergency contraception, such as not wanting to give women mixed messages about ongoing methos of contraception. In addition, research indicates that many physicians may be waiting for their patients to raise the issue. This leads Camp to conclude, "That's why it is critical to let women know they can still prevent pregnancy after unprotected sex, if they act quickly."
The two pharmaceutical companies that make emergency contraception market directly to consumers, but because these companies are very small, efforts to promote the products are limited and focused where they might see results more quickly, such as on college campuses. Given this landscape, advocates for emergency contraception have stepped in to educate women about their options. On March 20, 2002, the Reproductive Health Technologies Project (RHTP), joined by more than 100 medical and women's health groups, launched the "Back Up Your Birth Control" campaign to "raise awareness and position emergency contraception as a commonsense back-up method," says Kirsten Moore, president of RHTP. "We asked women to initiate conversations with their doctors. We asked doctors to talk to the women they see, pharmacists to stock dedicated products and organizations to make emergency contraception a priority in their outreach efforts. We reached more than 25 million Americans through news coverage, which overwhelmingly approached emergency contraception as an important consumer health issue."
Two other organizations that have taken a lead in educating women are the Association of Reproductive Health Professionals and the Office of Population Research at Princeton University. Together these organizations maintain the Emergency Contraception Website, which provides visitors with information, as well as the names of providers in their geographic area. The Web site was launched in 1994 by a small group of leaders in the field of reproductive health who recognized that promoting emergency contraception was perhaps the single most important unexploited strategy for reducing unintended pregnancy. Over the years, the number of visits to the site has grown from about 98,000 in 1997 to more than 365,000 in 2001. In 1996, organizers established the Emergency Contraception Hotline to accompany the Web site; both are confidential and available 24 hours a day in English and Spanish.
Support for Government Involvement
Research indicates that once people understand what emergency contraception is, the overwhelming majority are supportive and believe that couples should be told about the method. According to a July 2002 survey conducted by Peter D. Hart Research Associates on behalf of RHTP, two-thirds of voters think that government involvement, as a way of reducing the number of unintended pregnancies, is a good idea. In addition, three-fourths favor legislation aimed at expanding public health information about emergency contraception (see chart b). When asked why they favor government involvement, over 70% of voters reported that they consider the 72-hour window of effectiveness a compelling reason for women to know about the back-up option in advance of an emergency situation.
| chart b Strong Support |
|---|
| Voters informed about emergency contraception say they would support legislation expanding information about the method and its availability. |
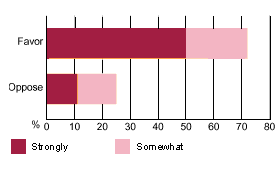 |
|
Source: Poll conducted by Peter D. Hart Research Associates for Reproductive Health Technologies Project, July 2002. |
On March 6, 2002, Rep. Louise Slaughter (D-NY) and Sen. Patty Murray (D-WA) introduced a bill that would allot $10 million a year for five years for an information and education campaign on the safety, efficacy and availability of emergency contraception. The campaign would attempt to reach the medical and public health communities, as well as the general public, working through the Health Resources and Services Administration and the Centers for Disease Control and Prevention. "We have the opportunity to fundamentally change the way we think about contraception in this country," said Slaughter when the bill was introduced. "The time has come for doctors to begin educating their patients on how to further prevent unwanted pregnancies and to make women aware of the options available to them."
Action on the Slaughter-Murray bill in this Congressional session is considered highly unlikely, but advocates are concerned that inaction could have decidedly negative effects. Trussell warns that without a sustained, national education campaign, women will remain unaware that a postcoital method exists and physicians will continue to wait for women to ask. "This is what I call a 'clinical bottleneck,'" he says. "Without a sustained awareness campaign, emergency contraception will remain only a potential, not reality."
Republished from an article by Heather Boonstra in the October 2002 issue of The Guttmacher Report on Public Policy. The article was supported in part by a grant from the Prospect Hill Foundation. The conclusions and opinions expressed in this article, however, are those of the author and The Alan Guttmacher Institute.
*This article is limited to a discussion of hormonal emergency contraception, which is the most commonly prescribed method of postcoital contraception in the United States. There are, however, other methods that exist or are being tested. The copper intrauterine device, if inserted within five days of unprotected intercourse, is a highly effective method with failure rates of less than 1%. Although this method is used infrequently in the United States, it is used extensively in Europe. Trials are also underway in the United States to evaluate the safety and efficacy of certain antiprogestins, the family of compounds that prevent or stop ovulation and retard the development of the uterine lining, depending on when in a woman's menstrual cycle they are taken.